For those of you familiar with MBER-Biology, you'll recognize this and the coming "Red" models. There are a few changes to this unit in particular: we have modified the transition into the unit since we are returning to biology after a pretty deep dive into earth science in Continent Formation. For MBER-Bio users who have used the alternative Chemical Reactions - Electrolysis unit (using a Hoffmann apparatus to split water molecules), you'll notice that we no longer support that version of the model here on the Living Earth website. You can still work to integrate that version of the unit into this sequence if that's your preference.
Phenomenon
Living things must take in stuff, rearrange it, and give it off in a different form in order to survive. (This phenomenon is not presented as an anchor at the start of the unit. We instead start with questions. But this is the phenomenon for the chemical reactions model as it unfolds during the unit.)

Question
Big Question: How do living things meet the challenges of survival and reproduction?
Specific Question: Why do living things need food? How do we get matter and energy from food?
Model Ideas
- The model – addressing the question, “Why do we need to eat food?”
- We recognize that weneed food to give us the matter and energy we need to survive.
- But how does this work?
Ideas About Matter from Food
- Food has matter in the form of protein, carbs and fats- the same things we find our bodies are made of. (We also take in matter as oxygen and water.)
- Matter is conserved, neither created nor destroyed.
- Matter is rearranged in chemical reactions.
- Some of this matter is used in our body, but we take in much more matter than we need to use to grow or maintain body structures.
- Some of this matter (especially much of the water but also some indigestible material) basically passes through us.
Ideas About Energy from Food
- Food has energy in the form of calories.
- Energy is conserved, neither created nor destroyed.
- Energy is transformed in chemical reactions.
- Living things get energy by rearranging matter in the form of food and oxygen molecules.
- When the reactants have more potential energy than the products, energy is released in the reaction (“downhill” reaction).*
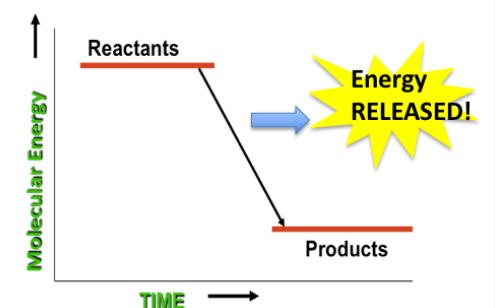
*Conversely, if the products have more energy, energy must be added to get the reaction to occur. We will wait to address this “reverse reaction” in later models.
Overview
All organisms need matter and energy to survive. Students will explore where that matter and energy comes from. They will build a model that explains how food can be transformed -via chemical reactions- to provide the matter and energy necessary for organisms to survive.
Transition in from Continent Formation: Now that the Earth has masses of land poking above the oceans, we return to thinking about life on the precipice of diversification. What challenges do living things face in Earth's shallow seas as well as those they face as they move onto the land?
This model is the first in a series of models (the Red Models Sequence, i.e. "matter and energy in organisms") that develops ideas around how organisms obtain what they need for survival and reproduction. In order to survive (and reproduce), organisms must obtain matter and energy. The chemical reaction model is the foundation for understanding how, by rearranging atoms in matter, organisms both obtain and store usable biological energy. There are two distinct stages of developing the model: (1) our bodies are matter transformation machines, and (2) matter transformations (called chemical reactions) can give off energy.
We start the unit by returning to biology and entering a conversation about what we need to take into our bodies in order to survive. As a class we then generate a rather comprehensive representation of ideas about our matter and energy needs called the “Inputs-Outputs-Uses” diagram. We revise this diagram several times in the first stage of developing the model, and then again in later models in the Red Models Sequence. We decide to first focus on one important input, food. In exploring food, we come to realize that it essentially gives us the matter (the “stuff”) and energy (the “doing”) we need, but we still have questions about how this works. Students learn about the molecular nature of the food we take in as well as the stuff our bodies put out. We pause to recognize that the outputs are made of the same elements as the inputs, but they are different in the molecular arrangement.
We infer that by rearranging these atoms organisms obtain the energy and matter they need to survive. This leads us to ask the question “How does rearranging atoms in our food give us energy?” This idea is explored in part by observing ethanol burning. We invoke conservation of energy to infer that the observable energy released in the reactions was somehow “in” the reactants. Some of the chemical potential energy of the reactants has now been transformed into visible energy. Because we know that energy is conserved, it follows that the products must therefore have less energy than the reactants. The same must happen with our bodies as some inputs are transformed into outputs. The reactants we take in (inputs) have more energy than the products we put out (outputs). In the process of rearranging the molecules, we free up the usable biological energy we need to “do stuff”. We end with a final segment where we take stock of what we know about how we get matter and energy from food.
* Note: A myriad of questions arise as students build the class representation of Inputs-Outputs-Uses. Many of these ideas are necessarily set aside while moving forward to make sense of the questions at hand. However, they are taken up in ensuing models. For example, questions about how plants obtain matter and energy (e.g. Do plants eat? Do plants eat sunshine?) usually arise throughout this segment. They are placed in the parking lot for now but can be used to transition into the photosynthesis model later.
Transition out to Cellular Respiration: Now that we have some understanding that we get the matter and energy we need by rearranging the molecules we take in through a series of chemical reactions, we are ready to further explore those reactions in order to make sense of organismal biology in the coming models.
Overall Time: 8-10 days
Advanced Planning
MATERIALS NEEDED (besides basic lab equipment and supplies):
See Resource "Burning Ethanol Teacher Guide" in Learning Segment Table above for more information. Materials required will depend on your class investigation of burning ethanol, most notably how you will test for the presence of potential products from the reaction.
You should plan to have all these resources on hand. (Most are likely already in your possession or in the possession of teachers at your school. Others are cheap and easy to purchase.)
- marshmallows/skewers or equipment to burn Cheetos
- ignition sources (matches might be ok, but better to have camping lighters with long handles)
- glass petri dishes (enough for however many groups you plan to have work semi-independently (under your supervision)
- 95% or 100% ethanol (95% is cheaper and your chemistry teacher next door likely has some)
- bromothymol blue (from above CO2 experiment)
- plastic containers with enough overhead to suffocate/extinguish the reaction without being exposed to the two-inch flames
- a watchglass or extra glass petri dish (chilled on day of use)
1: Challenges on the Land / The IOU Diagram
Overview: Following the exploration of continent formation in the last unit, we turn back to biology by wondering a little about the challenges life faced in the shallow oceans and as it began to crawl onto land. We broaden the conversation to consider, “What do organisms need to take in to survive? What do they use it for and what is given off?” Students work to generate a representation of inputs-outputs-uses.
What we figured out... We have reoriented to biology and have brainstormed a number of challenges all life faces in order to survive and reproduce. We’ve come to represent our thinking as a class on an “inputs-outputs-uses” poster that reflects our current understanding of the needs of organisms.

2: Why Do We Need Food?
Overview: We shift our broader conversation about an organism’s inputs and outputs to consider just one in particular--food. And then we probe: WHY do we die if we don’t eat? (Really, why?) We finish by offering some initial ideas.
What we figured out... We have paused to refocus our attention on a specific organismal need—food. And we have a refined question, “Why do we need food? Or, why do we die if we don’t eat?” This becomes the Driving Question for the remainder of the unit.

3: What Is Food / The Calorie King
Overview: Students engage in conversation about what is actually in food. After they have described the major components—carbohydrates, fat, and protein, they make a connection to the composition of humans and other organisms. We recognize that “we are what we eat”.
What we figured out... We learned about the composition of food, that both food and our own bodies are made up of carbs, proteins and fats. But what are carbs, fats and proteins made of?

4: Molecular Nature of Food
Overview: We next go deeper to look at the elements that make up those molecules. Ultimately, we will recognize that mostly the same basic four elements are going into and out of our bodies.
What we figured out... We went deeper in our understanding of the molecular composition of food by studying the structure of biomolecules.

5: Exploring Matter and Energy
Overview: We pause to pose the questions, “What is matter? What is energy?” The class generates a common understanding of these ideas before moving forward in exploring their roles in the survival and reproduction of organisms.
What we figured out... We have some basic ideas about matter and energy and a bit of common language. We’ve also discussed the Laws of Conservation of Matter and Energy which we will leverage later.

6: Outputs Are Rearranged
Overview: We next explore the molecular nature of the outputs, setting the stage for the identification of a key phenomenon: What comes out is not exactly the same as what went in. We are matter/molecular transforming machines.
What we figured out... We’ve recognized that in our bodies matter is rearranged. This leads us to ask, “Why?”

7: Why Transform Matter?
Overview: We are now trying to understand why we transform matter. This is further motivated start by noticing the majority of the matter we take in is not incorporated. Why bother moving all this matter through our bodies? Through discussion, we come to figure out it’s all about energy.
What we figured out... We’ve recognized that matter transformation must have something to do with energy.
8: Burning Food
Overview: We recognize that we still don’t know how we get the energy from matter transformations. Since calories are a measure of the energy in food and we burn calories, we explore what happens when we burn food. We recognize that there is an input-output transformation in burning a Cheeto or marshmallow, but the inputs and outputs are complex. If we want to track matter and energy in a chemical reaction, we need a simpler system.
What we figured out... We’ve developed some ideas around food as fuel and burning as an important chemical reaction. However, the inputs and outputs are complex. If we want to track matter and energy in a chemical reaction, we need a simpler system. Therefore, we are moving on to consider the burning of ethanol, a pure fuel source made of C H and O (just like our food).
9: Burning Ethanol - Tracking Matter
Overview: In burning ethanol, we first track the rearrangement of molecules. What are the inputs and outputs? A discussion of burning leads us to the testable idea that oxygen is the other input. Recalling the conservation of matter, we generate ideas about what the outputs might be and test these ideas.
What we figured out... We have a balanced equation for burning ethanol, but we still need to figure out what is happening with the energy.

10: Burning Ethanol - Tracking Energy
Overview: We move on to discuss energy. What does it look like before, during and after the reaction? If energy is conserved, where does it come from? Here we recognize the transformation of energy from potential to other forms through use of an analogy. Students generate a representation of what is happening with energy in chemical reactions like burning. The clear inference is that molecules have different inherent energies. Since energy is released here, the reactants must have had more potential energy than the products.
What we figured out... We know that energy is transformed from potential to visible energy in the burning reaction, and we finally have a model for how energy can be released during chemical reactions.

11: Model Summary and Application
Overview: We take stock of (1) what we have figured out and (2) what we are still wondering about, returning to our driving question. We apply our understanding to a Challenge Question about a coma patient.
What we figured out... We’ve finalized our model for chemical reactions and have partially answered our driving question, motivating further exploration of Cellular Respiration.
